CO2 Enhanced Extraction Shale Gas
In recent years, shale gas has changed the energy resource landscape in the United States and it will play an important role for foreseeable future. While having unconventional resources such as shale gas and tight oil have been great for the US energy demand, the environmental concerns have also been raised. In China, their shale gas and oil are found in much deeper regions and they are located where water is a limited resource. Thus, we have been investigating the use of CO2 as an alternative fracking fluid for extracting these unconventional resources.
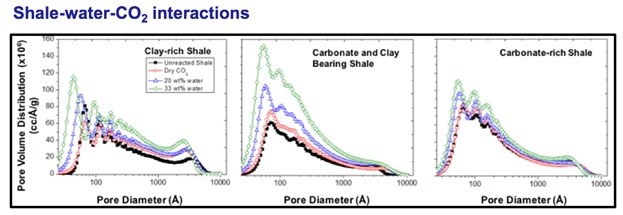
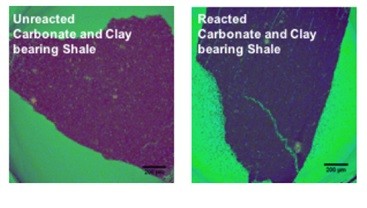
CO2-shale-water interactions have shown that CO2 can rapidly react with shale and other rocks depending on their mineralogy and the presence of water. As shown in below figures, our synchrotron study has shown that there is interesting development of pore network as CO2 reacts with carbonate and clay-rich shale. Fundamental research is needed to understand the shale-water-CO2 interactions in order to design an effective enhanced extraction process for shale gas and tight oil.
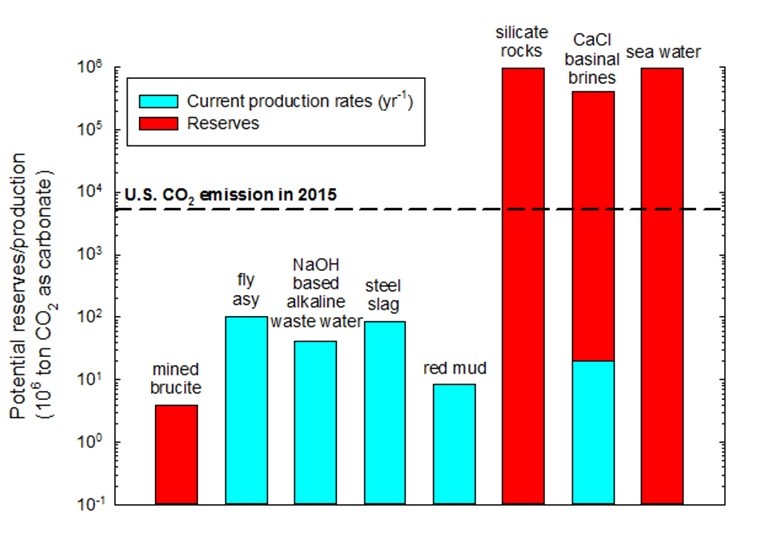
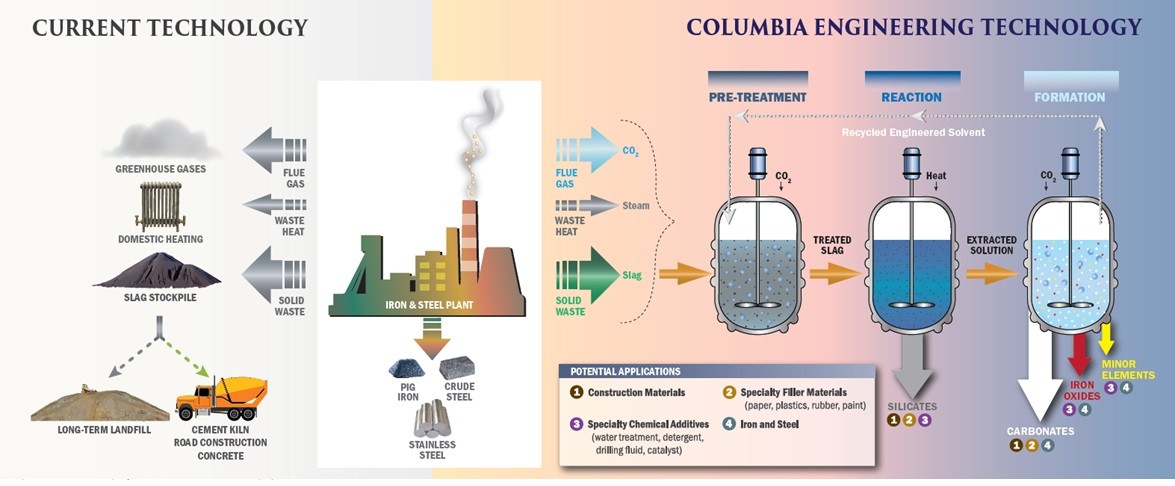
In order to address both CO2 emission and solid waste issues, our research group has been developing mineral dissolution and carbonation technologies for various industrial wastes, while focusing on the simultaneous extraction of other valuable minor components such as rare earth elements with other physical and chemical processes. While these reactions are thermodynamically favorable, the reaction kinetics are slow and the separation of products is very difficult when trying to produce high value products. Thus, a new approach to waste management that can close the material, water and energy cycles within the urban industrial, social, environmental, and economic systems is highly desired to achieve urban sustainability.
We have been working on the carbonation of industrial wastes, particularly steel slags, for a number of years. Many waste streams have high alkalinity, and thus, they can react with CO2 and form thermodynamically stable solid carbonates. Furthermore, many industries producing these alkaline wastes are also point sources of CO2 making carbon mineralization a further attractive reprocessing option while providing a great opportunity to reduce the carbon footprint. By diversifying the carbonation products from mineral carbonation and extracting valuable components from the wastes, the overall alkaline waste carbonation process can become more economically feasible.