NOHMs CO2 Capture
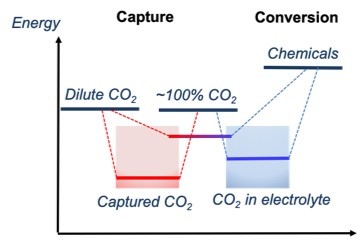
Most of the recent CO2 conversion research has been focused on catalyst development. While the role of catalysts is very important, it is also important to consider other challenges such as the solubility of CO2 in the electrolyte. Water is not used as a CO2 capture solvent because the CO2 solubility is low. Thus, the dissolution of captured CO2 in the electrolyte is limited and this will limit the overall CO2-to-chemicals and fuels process. Thus, in my research group, we have been developing dual-functional materials that can capture and convert CO2 in a single reactive medium.
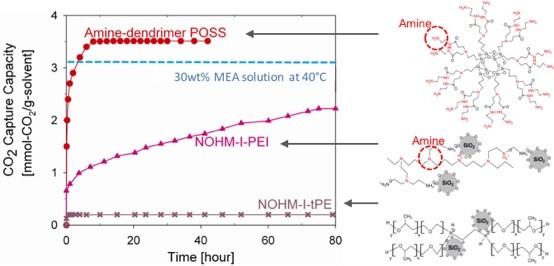
Liquid-like NOHMs (Nanoparticle Organic Hybrid Materials)are an emerging class of self-suspended nanoparticles with high CO2 capture capacity, particularly when functionalized with amine. NOHMs are comprised of a polymeric canopy tethered to surface-modified nanoparticles and have great thermal stability and negligible vapor pressure. NOHMs can be designed with unique nanoscale structures to shape the way CO2 is incorporated into the NOHMs via entropic effects. We have been able to modify the viscosity of NOHMs by adding a secondary fluid. We synthesize various solvent mixtures containing NOHMs in order to incorporate different CO2-targeting functional groups. The ionic conductivity can be tuned via changing the surface chemistry of the core or adding different functional groups to the canopy.
We have also discovered that NOHMs could be a novel electrolyte with high CO2 solubility. The preliminary synchrotron study at ANL has revealed that their unusually high conductivity may be due to a unique fractal structure exhibited by NOHMs in the bulk fluid. Thus, we envision that NOHMs may effectively host both CO2 capture and conversion reactions. These dual-purpose materials will allow innovative reaction pathways and reduce the overall parasitic energy consumption.