In terms of CO2 capture using solid sorbents, Metal Organic Frameworks (MOFs) are one of the most studied materials in recent years. MOFs are very interesting and have many positive properties for CO2 capture. But they also suffer from a number of challenges such as weak tolerance to water and long synthesis time. Furthermore, once MOF crystals are produced, it is important to develop methods to deliver MOFs to capture CO2. Currently, MOFs are incorporated into advanced membranes and our collaborator has also produced polymer beads that contain MOFs. While these techniques are interesting, the fabrication of these hybrid systems needs to be further developed.
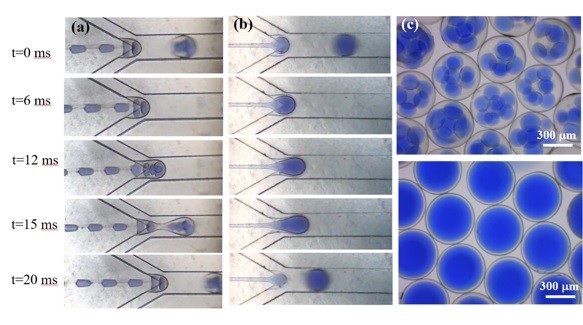
In our research group, we have developed a microfluidic device and encapsulation technique that can directly synthesize and package MOFs within the gas permeable shell for various energy and environmental applications (e.g., CO2 capture and other gas separations and conversion). As shown in the below figure, for the first time, we were able to directly synthesize MOFs crystals in droplets enclosed in a UV-cured shell. Once produced, these MOFs-bearing particles (in the range of 200-500 micron size) can easily be used in various gas separation units (e.g., fixed beds and fluidized beds) with the ease of handling.
In addition, we are currently investigating to develop this system to co-encapsulate MOFs and catalysts that can simultaneously capture gases and convert them into products (e.g., combined CO2 capture and conversion to chemicals and fuels).
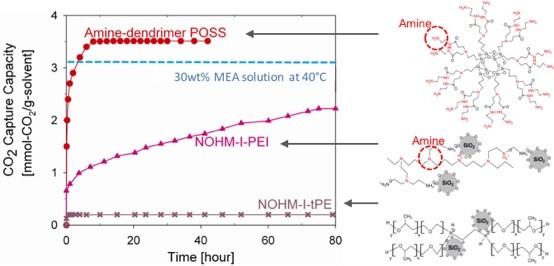
Liquid-like NOHMs (Nanoparticle Organic Hybrid Materials)are an emerging class of self-suspended nanoparticles with high CO2 capture capacity, particularly when functionalized with amine. NOHMs comprise a polymeric canopy tethered to surface-modified nanoparticles and have great thermal stability and negligible vapor pressure. NOHMs can be designed with unique nanoscale structures to shape the way CO2 is incorporated into the NOHMs via entropic effects.
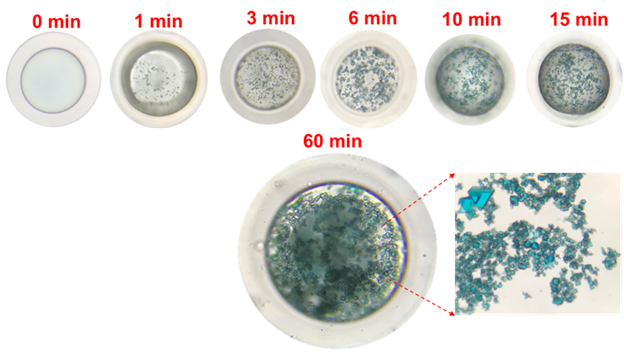
While NOHMs and other anhydrous or water-lean CO2 capture solvents (e.g., Ionic liquids) are interesting, they are challenged by their high viscosity. Thus, there have been significant efforts in addressing the high viscosity issue. One of the innovative techniques recently developed by LLNL team is encapsulation. By packaging the viscous CO2 capture solvent in a gas permeable shell, you can now use these CO2 capture solvents as a particulate system with more flexibility in reactor design (e.g., fixed bed to fluidized bed). At Columbia University, for the first time we have developed a microfluidic device that can continuously produce these encapsulated solvents. These encapsulated solvents would not evaporate since they have a negligible vapor pressure. The size and thickness of the shell can be tuned as well as the chemistry of NOHMs for the optimized gas separations.
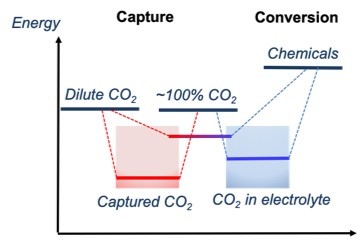
Most of the recent CO2 conversion research has been focused on catalyst development. While the role of catalysts is very important, it is also important to consider other challenges such as the solubility of CO2 in the electrolyte. Water is not used as a CO2 capture solvent because the CO2 solubility is low. Thus, the dissolution of captured CO2 in the electrolyte is limited and this will limit the overall CO2-to-chemicals and fuels process. Thus, in my research group, we have been developing dual-functional materials that can capture and convert CO2 in a single reactive medium.
Liquid-like NOHMs (Nanoparticle Organic Hybrid Materials)are an emerging class of self-suspended nanoparticles with high CO2 capture capacity, particularly when functionalized with amine. NOHMs are comprised of a polymeric canopy tethered to surface-modified nanoparticles and have great thermal stability and negligible vapor pressure. NOHMs can be designed with unique nanoscale structures to shape the way CO2 is incorporated into the NOHMs via entropic effects. We have been able to modify the viscosity of NOHMs by adding a secondary fluid. We synthesize various solvent mixtures containing NOHMs in order to incorporate different CO2-targeting functional groups. The ionic conductivity can be tuned via changing the surface chemistry of the core or adding different functional groups to the canopy.
We have also discovered that NOHMs could be a novel electrolyte with high CO2 solubility. The preliminary synchrotron study at ANL has revealed that their unusually high conductivity may be due to a unique fractal structure exhibited by NOHMs in the bulk fluid. Thus, we envision that NOHMs may effectively host both CO2 capture and conversion reactions. These dual-purpose materials will allow innovative reaction pathways and reduce the overall parasitic energy consumption.
While CO2 utilization would play an important role in our sustainable energy future, due to the unprecedented amount of CO2 emission, a large fraction of the captured CO2 should be stored in order to manage the atmospheric CO2 level in foreseeable future. There are a number of storage options for CO2 and some includes the in-situ carbon mineralization via the reaction between CO2 and silicate minerals. In the past, while it is thermodynamically favored, the carbon mineralization reaction was considered to be too slow. Basalt is one of the most abundant rock containing Ca and Mg but low in its mineralization capacity compared to magnesium silicate minerals such as olivine and serpentine. But recently our colleagues at Columbia University have shown that CO2 can rapidly be mineralized in basaltic formation (white carbonate crystals shown in the photo from Iceland CarbFix project – we were involved in their tracer test).
In my research group, we have actively been working on CO2-mineral-water interactions for more than a decade. While in order to inject most amount of CO2 in any reservoir you want to remove all the water to create more empty space. But the reactivity of minerals and rocks in geologic formations including basalt would significantly be reduced when only dry CO2 is interacting with minerals. Thus, the investigation on the optimized amount of water in CO2 injection into basalt or Mg-silicate formations would be one of the important research questions. We have a unique high pressure differential bed reactor system that can measure the fast reaction kinetics far from equilibrium creating the in-situ geologic systems. Through these fundamental studies, we are currently developing water alternating gas (WAG) method that can maximize the in-situ carbon mineralization

The conversion of magnesium silicate minerals to solid carbonates is an environmentally safe and permanent option for capturing and storing carbon dioxide. The technology is currently challenged because the kinetics of magnesium extraction at low temperatures are prohibitively slow without energy intensive pretreatments. Progressively slowed kinetics are thought to be caused by a silica-rich passivation layer; as magnesium dissolves, the remaining silica accumulates on the particle surface and creates a barrier that slows further dissolution of magnesium from the mineral surface.
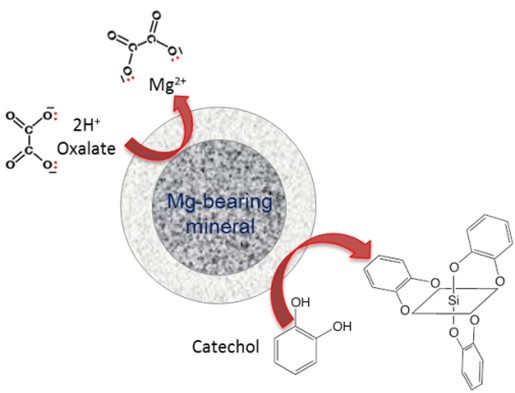
In our group, we have been developing a chemical and biological catalytic system that can significantly accelerate silicate mineral dissolution. A combination of chelating agents, catechol and oxalate, were investigated as chemical additives for countering the effect of this passivation layer. Under acidic conditions, oxalate and catechol together accelerated magnesium dissolution by modifying the pore structure of the passivation layer, while not impeding the subsequent conversion of magnesium to carbonates. The role of an enzymatic catalyst, carbonic anhydrase, on the CO2 hydration was also investigated to enhance the precipitation of mineral carbonates at moderate pH conditions.
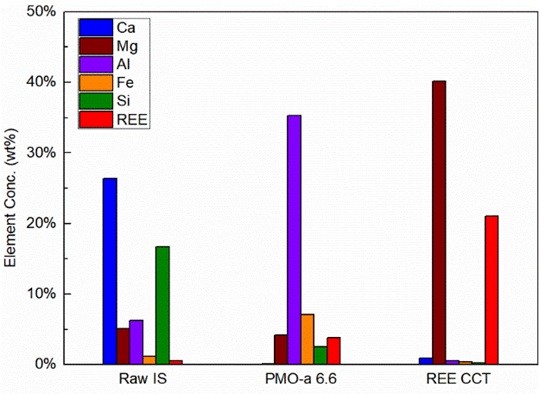
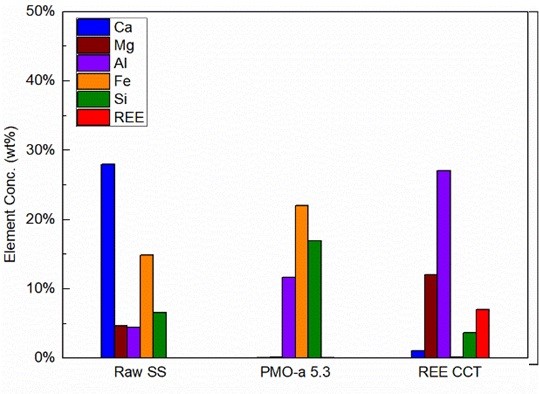
When employed as the feedstock of the mineral carbonation process, the iron and steel slags can be converted into valuable carbonate materials, while contributing to the mitigation of CO2 emissions. Considering iron and steel slags can contain certain quantities of rare earth elements (REEs) depending on the origins of the raw materials and the manufacturing process, the integration of the recovery of REEs with the slag carbonation process can also help to mitigate the supply risk of REEs by developing these unconventional REE resources derived from the waste streams. Considering the high level of process integration, high utilization efficiency of the energy and the materials can be expected.
In our group, we have been studying the fate of REEs in iron and steel slags during slag carbonation process. We have also been developing methods and techniques to recover and concentrate REE in the slags as a coproduct of the slag carbonation process. Currently, rare-earth concentrates (REE CCT) and precipitated calcium carbonates (PCC) of high purities have been successfully produced from the slags with our process. In order to further enhance the extraction of valuable elements in the slags and separation of different substances during the process, different physical and chemical methods are being investigated, including froth flotation, gravity separation, magnetic separation, acid and base treatment, solvent extraction, and ion-exchange chromatography.